The UC Davis Interdisciplinary Center for Plasma Mass Spectrometry has two labs on the Davis campus, one in the Plant & Environmental Sciences building and one in the Earth & Physical Sciences building. The equipment housed in these labs can perform various types of analysis, meeting the needs of a wide range of research projects both on the Davis campus and outside the university community.
Facilities
Inductively coupled plasma (quadrupole) mass spectrometry (ICP-MS)
In ICP-MS, a sample in the form of an aerosol is introduced into high temperature argon plasma by a carrier gas. Aerosol particles are generated either by nebulizing a sample solution or by ablating a solid sample with a laser. The temperature of the centerline of argon plasma reaches ~6000 to 10000 K such that the sample is vaporized, atomized, and ionized (mostly singly charged positive ions with small amounts of doubly charged ions and molecular ions). The ions are extracted from the plasma into a high vacuum (~ 10-5 torr) region and focused by the ion lens system. Ions are then separated according to their mass/charge ratio by the quadrupole mass analyzer and detected by an electron multiplier.

The UCD ICPMS lab in the Plant & Environmental Sciences building has two Agilent Technologies quadrupole ICP mass spectrometers: a single quadrupole 7500ce and a triple quadrupole 8900. The 7500ce instrument has an octopole component called a collision/reaction cell (CRC) between the ion lens system and quadrupole, while the 8900 instrument also has an additional quadrupole after both the ion lens system and the second quadrupole which serves as the CRC – a total of three. The 7500ce instrument’s CRC can use helium or hydrogen gas to reduce common molecular ion interferences for several elements. Helium gas can be used to decelerate molecular ions such as chlorine oxide and argon chloride through collisions, reducing their interference with elements such as V and As. Hydrogen gas also reduces molecular ion interference through collisions, but the hydrogen can also react with argon oxides and argon dimers to selectively reduce their interference with elements such as Fe and Se. The CRC is a useful tool for reducing or eliminating interferences that cannot be removed using standard methods.
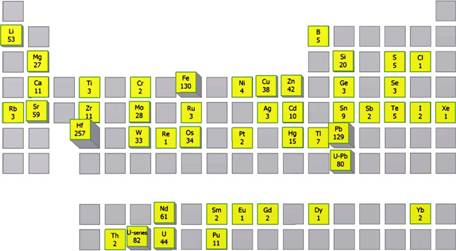
When combined with the triple quadrupole technology featured in the 8900, the CRC can also be used for analysis of several notoriously difficult elements with otherwise very high detection limits including Si, P, S, and Cl. This is achieved by using hydrogen, oxygen, or ammonia gas in the CRC to react or combine with pre-cursor single or molecular ions that can be selected using the first quadrupole, and the single ion or molecular products can then be discriminated from one another using the second quadrupole.
The 8900 instrument also features a high matrix introduction (HMI) system that enables the analysis of high Total Dissolved Solids (TDS) samples and allows for ‘no-dilution’ analysis of natural waters in most cases. The ‘inert kit’ option is also available for analysis of concentrated acid solutions including samples containing significant amounts of HF. The triple quadrupole technology and improved detector and vacuum systems have lowered the background and increased the sensitivity and the linear dynamic range for all analytes.
Multiple-Collection Inductively Coupled Plasma Mass Spectrometry (MC-ICP-MS)

The UCD ICPMS lab in the Earth & Physical Sciences building utilizes a Nu Plasma HR (Nu032) multiple-collection high-resolution double-focusing mass spectrometer ICP system capable of determining isotope ratios with external precision on the order of 10-20 ppm. This instrumentation better constrains isotopic proportions in natural samples than possible with quadrupole ICP-MS or single-collector HR-ICP-MS systems.
Multiple collection inductively coupled plasma mass spectrometry (MC-ICP-MS) precision is similar to that achieved by thermal ionization mass spectrometry (TIMS) but has a tremendous speed advantage over TIMS since analyses can be performed within 20 minutes per sample (depending on the target analytes) compared to hours per sample typically required for TIMS. In addition, due to the ionization efficiency of the high temperature ICP ion source, MC-ICP-MS can be used to study both traditional and nontraditional isotope systems for a wide range of applications.